http://http://www.youtube.com/watch?v=99wPiMb-k0o
Here is a great Video that I found that explains the basic infromation that every aspiring scientist needs to know about zinc. Enjoy!
Monday, December 7, 2009
Saturday, December 5, 2009
Physical and Chemical properties of the Element
Zinc has different hues of colour, some of them being blue, grey or a whitish shade, at times it is shinny in luster. It is very brittle(easily broken) at room temperature ,but when it heated between the temperatures of 100 and 150 Celsius it becomes malleable (can be hammered or flattened into sheets.) Zinc is a conductor of electricity but it sometimes is not depending on how much of it is used. Zinc also burns in air at very high temperatures called high red heat, it is then followed by the production of white clouds that are harmful when inhaled. The density of zinc is 7.140 g/ml which means that it will not float in water and will sink. Zinc is a very heavy metal compared to others.
Most of the time zinc is taken from sulfide ores. These ores are cooked in industrial plants at very high temperatures for a long period of time to form zinc oxide(Zn O). A process where carbon is added is then executed so that the zinc oxide is converted to zinc in metal form.
Most of the time zinc is taken from sulfide ores. These ores are cooked in industrial plants at very high temperatures for a long period of time to form zinc oxide(Zn O). A process where carbon is added is then executed so that the zinc oxide is converted to zinc in metal form.
The Common Uses of Zinc

Over 7 million tons of zinc are produced yearly around the world. Although zinc has many uses its most common use is used for galvanizing steel to protect it from rusting and corroding. Some other uses fro zinc is for the production of brass , about 19% of the world's zinc is used for this. Another 165 is used for the production of alloys (like the penny experiment we did in class), these are then supplied to the die and casting companies around the world. Zinc is also used for common purposes such as roofing, gutters and pipes in homes.
For plants ,animals and us humans zinc is an important mineral for growing and developing in fact it plays such and important role in our growth and development that about 2 to 3 grams of it can be found in our bodies. About 300 of our enzymes depend on our intake of zinc. If you were to go and find zinc in our bodies you would find it in almost every part of the body that includes the organs, tissues and bones. Our bones hold most of the body's zinc at a whopping 90%. So if you don't want weak bones start taking the required amounts of zinc your body needs.* Be very careful when taking zinc deficiency supplements because too much of it can seriously hurt you!*
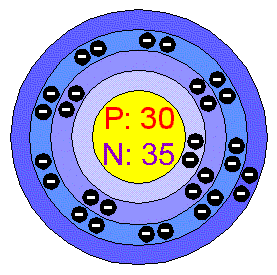
The Atomic Structure of Zinc
Basic Information About Zinc

Here is some basic information about zinc.
Its symbol on the periodic table is Zn. Its Atomic mass (how much it weighs atomically) is 65. Its atomic number on the periodic table is 30. It begins to boil at the very smoldering temperature of 907 degrees Celsius.The number of electrons is equal to the number of protons so zinc has 30. Zinc has 35 neutrons. We would classify zinc as a transitional metal meaning that it can exhibit several oxidation states , there are other metals that are transitional but the most note worthy are iron , nickle and cobalt.( sorry, even if zinc is part of the transitional family it is not one of the note worthy but it should be!) If you were to chip away at a piece of zinc and put it under a microscope you would find that its crystal structure is hexagonal which is very cool if you get a chance to take a look.
The History of Zinc

The history of Zinc.
Centuries before zinc was discovered in the metallic form, its ores were used for making brass
and zinc compounds, its ores were also used for healing wounds and sore eyes. It is believed that the Romans first made brass in the time of Augustus.
Zinc was discovered in 1746 by Andreas Sigismund Marggraf of Germany by isolating the pure metal through heating calamine and charcoal. It comes from the German word "zink" for tin.
Subscribe to:
Comments (Atom)